Click-to-Release
OUR TECHNOLOGY
Enabling universal, chemically controlled, on-target activation of antibody-drug conjugates (ADCs) and immunomodulators, as well as off-target deactivation of radiopharmaceuticals
While there has been incredible progress in recent years, the impact of many systemic therapies has been limited by their therapeutic index – the measure of the effective dose that can be administered to a patient relative to the drug’s safety in the body.
Tagworks has built a unique technology known as Click-to-Release, based on its invention of the pyridazine elimination reaction, so far the only bioorthogonal cleavage reaction that is selective and fast enough for clinical use in vivo. After its discovery, the team further developed the chemistry into a broadly applicable platform technology for systemic therapies.
Click-to-Release can capture the therapeutic potential of highly potent systemic therapies in multiple ways, including:
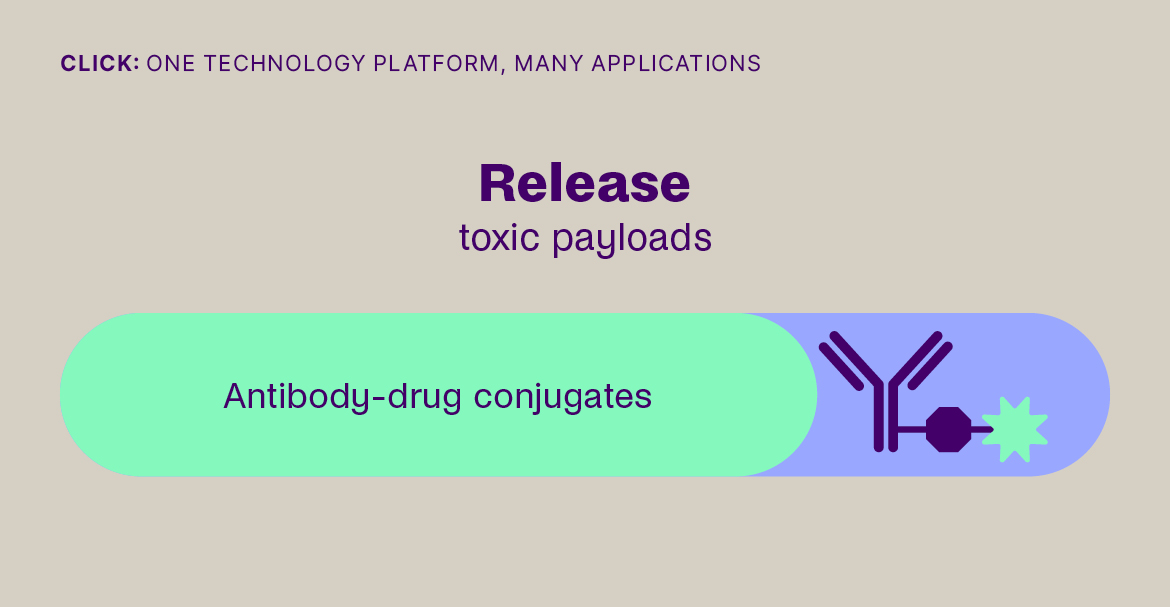
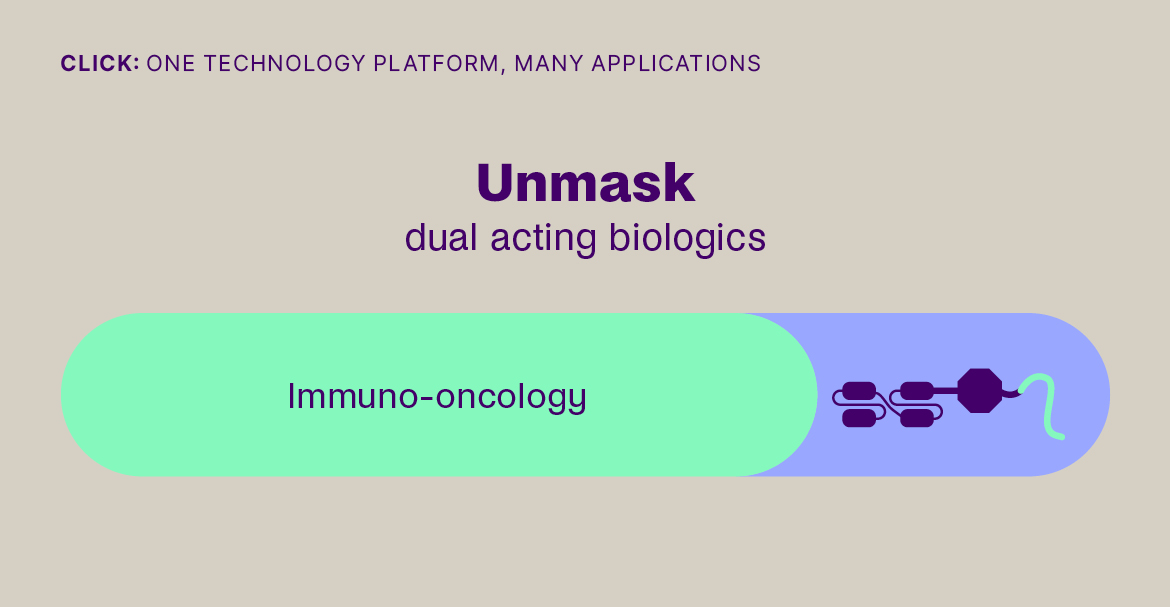
- By enabling their safe delivery to the tumor microenvironment and their controlled on-target activation, as with ADCs and masked immunomodulators, increasing on-target efficacy.
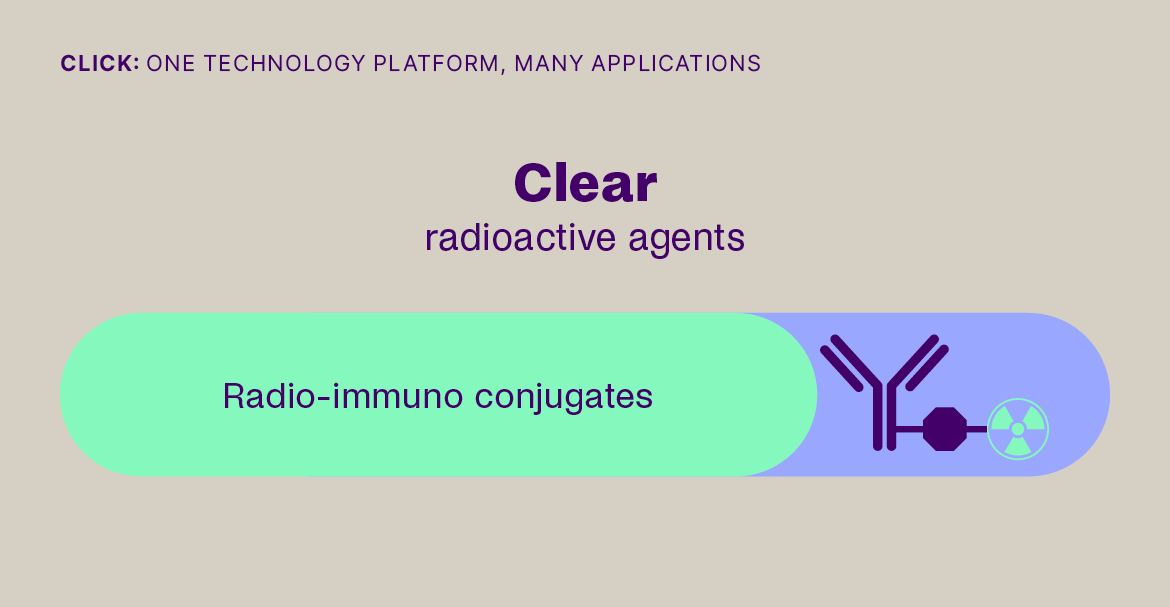
- By enabling off-target deactivation of radioimmunotherapy, by selective cleavage and renal clearance of the radiolabel from freely circulating radioimmunotherapeutics, decreasing dose-limiting toxicity in the bone marrow.
How our Technology Works
Click-to-Release is based on the sequential administration of a pair of molecules that only react (click) with each other, but not with any other molecule they encounter. One molecule comprises a trans-cyclooctene (TCO) linker bound to a payload and the other molecule is a tetrazine, which acts as the trigger. When the TCO linker and tetrazine trigger click together, a follow-on reaction is triggered that instantaneously releases the payload bound to the TCO linker.
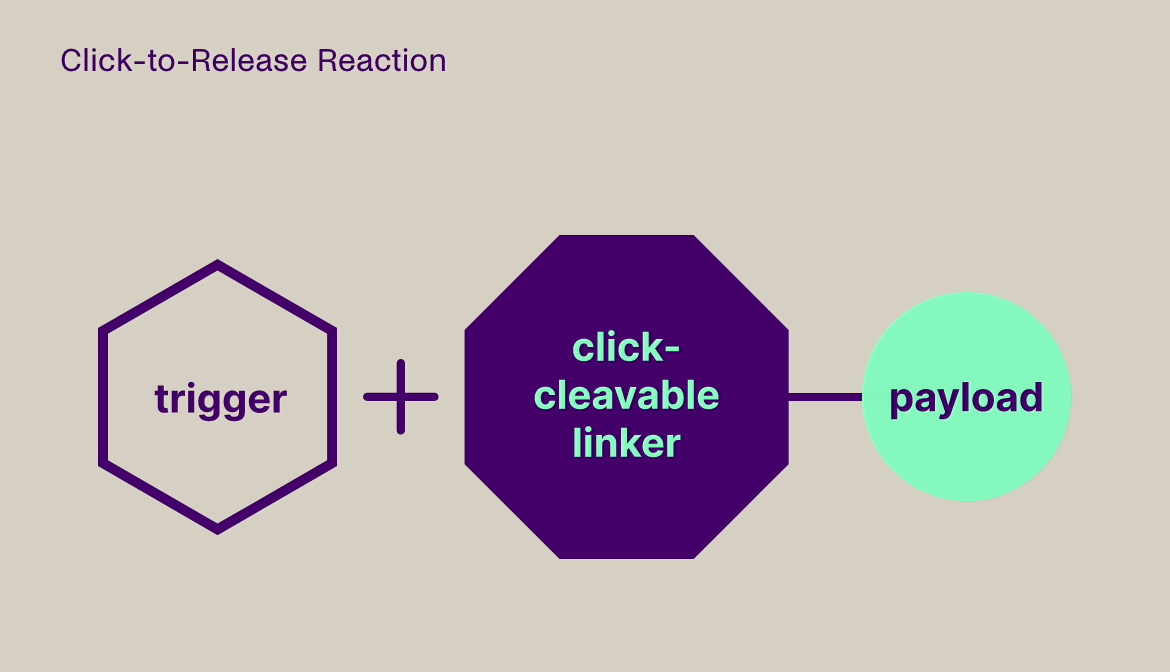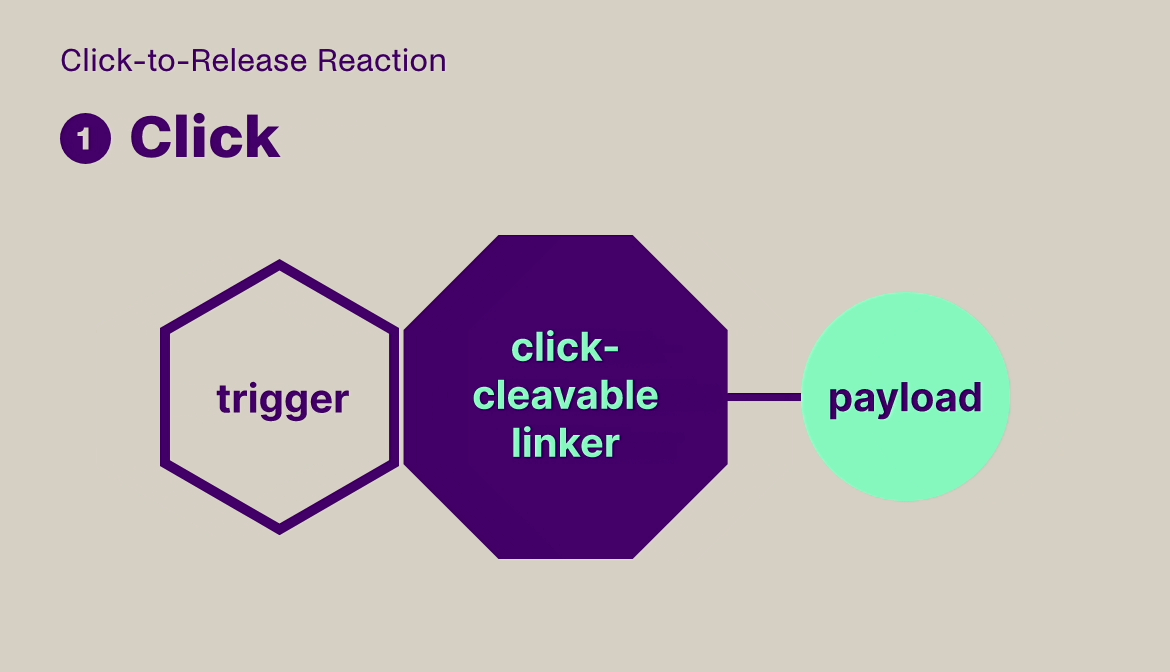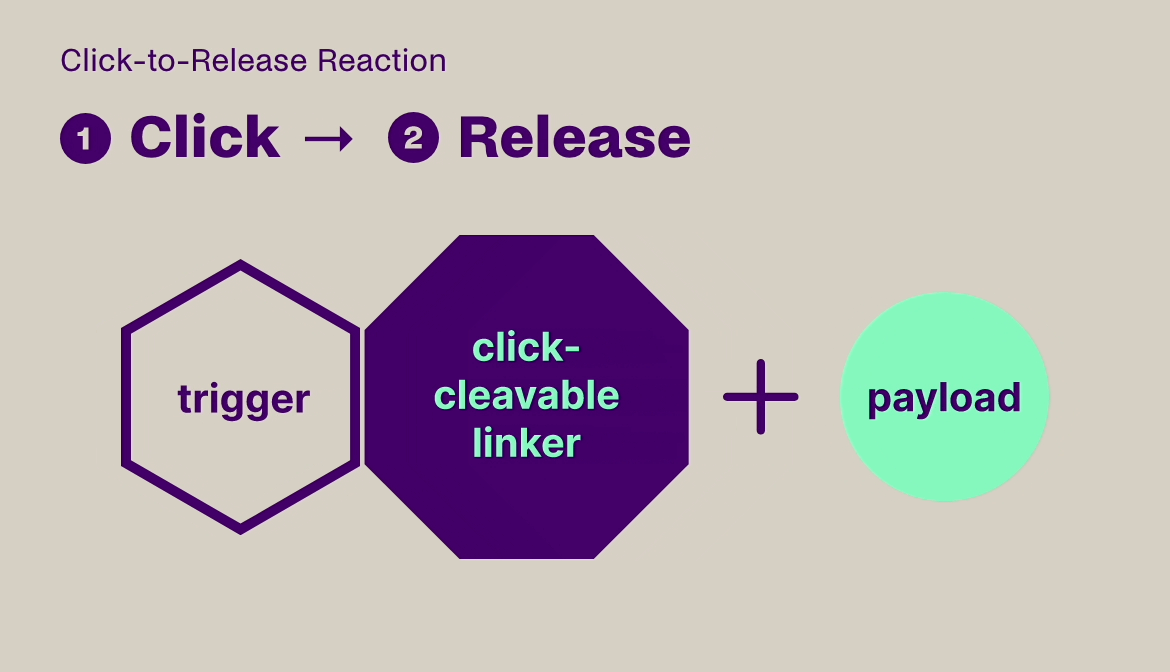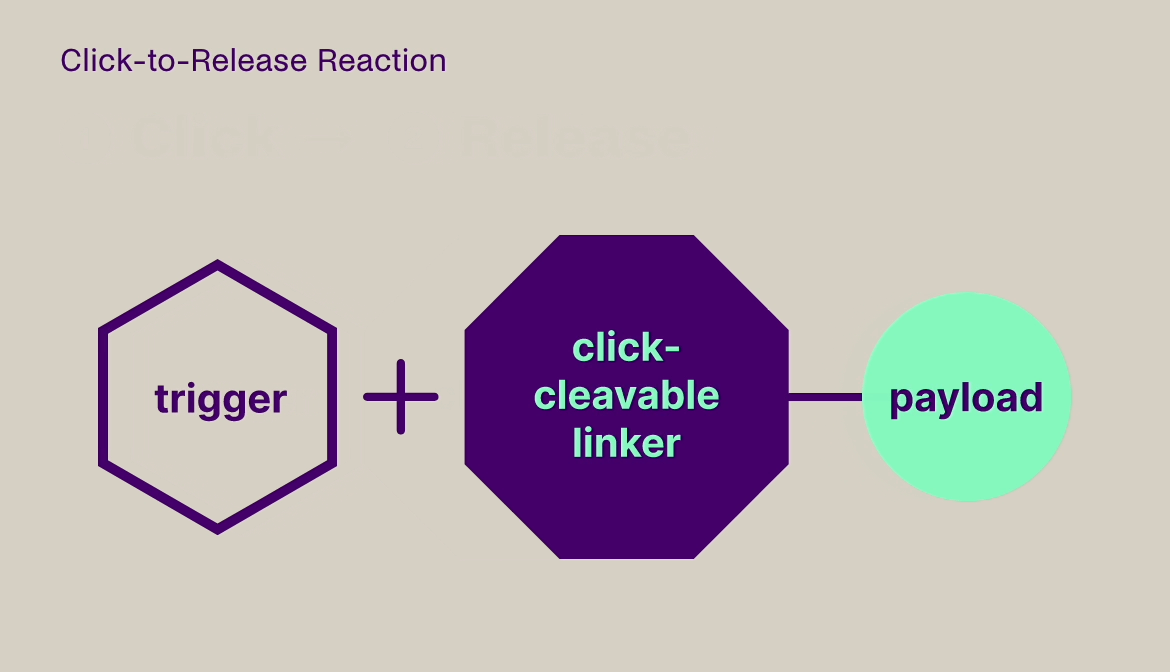
Our platform is compatible with small molecules, peptides, and larger biomolecules, such as antibodies and antibody fragments, and covers a wide range of toxin and immunomodulator classes. It can be used to release a payload from a targeting agent to unmask and activate a payload in the body, or to clear a payload from non-target tissues. This technology offers fast, universal & temporally controlled release that does not rely on currently used biological drug release mechanisms (e.g. proteases), thereby potentially increasing the application scope and therapeutic index for several therapeutic modalities. In addition, the chemically controlled release is designed to make therapies such as ADCs safer, due to the stability of the linker in the absence of the trigger.
Tagworks is initially leveraging its approach to expand the number of ADC targets. In addition to the lead ADC program, TGW101, the pipeline includes discovery phase programs in ADCs and radiopharmaceuticals.
Tagworks has a broad IP estate on the Click-to-Release reaction, covering wide range of chemical and application scope. In addition, Tagworks owns a broad IP portfolio on in vivo Click Conjugation (for pre-targeted radioimmunotherapy).
Publications
Toward realization of bioorthogonal chemistry in the clinic
Topics in Current Chemistry, 5 March 2025
Ortho-functionalized pyridinyl-tetrazines break the inverse correlation between click reactivity and cleavage yields in click-to-release chemistry
Communications Chemistry, 19 December 2024
Click-to-Release: Cleavable Radio-immunoimaging with 89Zr-DFO-Trans-Cyclooctene-Trastuzumab Increases Tumor-to-Blood Ratio
Theranostics, 9 July 2023
Curious about what we do? Get in touch!
Contact
T +31 85 800 8550
info@tagworkspharma.com
Address
Tagworks Pharmaceuticals BV
Toernooiveld 1
6525 ED Nijmegen
The Netherlands
Stay updated
© Tagworks Pharmaceuticals, 2025 | Terms of use | Design by Ape to Zebra