Lowering the hurdle to success against solid tumors
PIPELINE
Capturing the untapped therapeutic potential of ADCs and other systemic treatments
Tagworks’ lead program, TGW101, targets TAG72, a non-internalizing clinically validated pan-carcinoma target, present in several solid tumors, which cannot be effectively addressed with current ADC technologies. In addition, our pipeline includes discovery phase programs on other solid-tumor-targeted ADCs as well as radiopharmaceuticals, including a HER2-targeted radioimmunotherapeutic.
Our pipeline
Click-Cleavable ADCs: The Opportunity
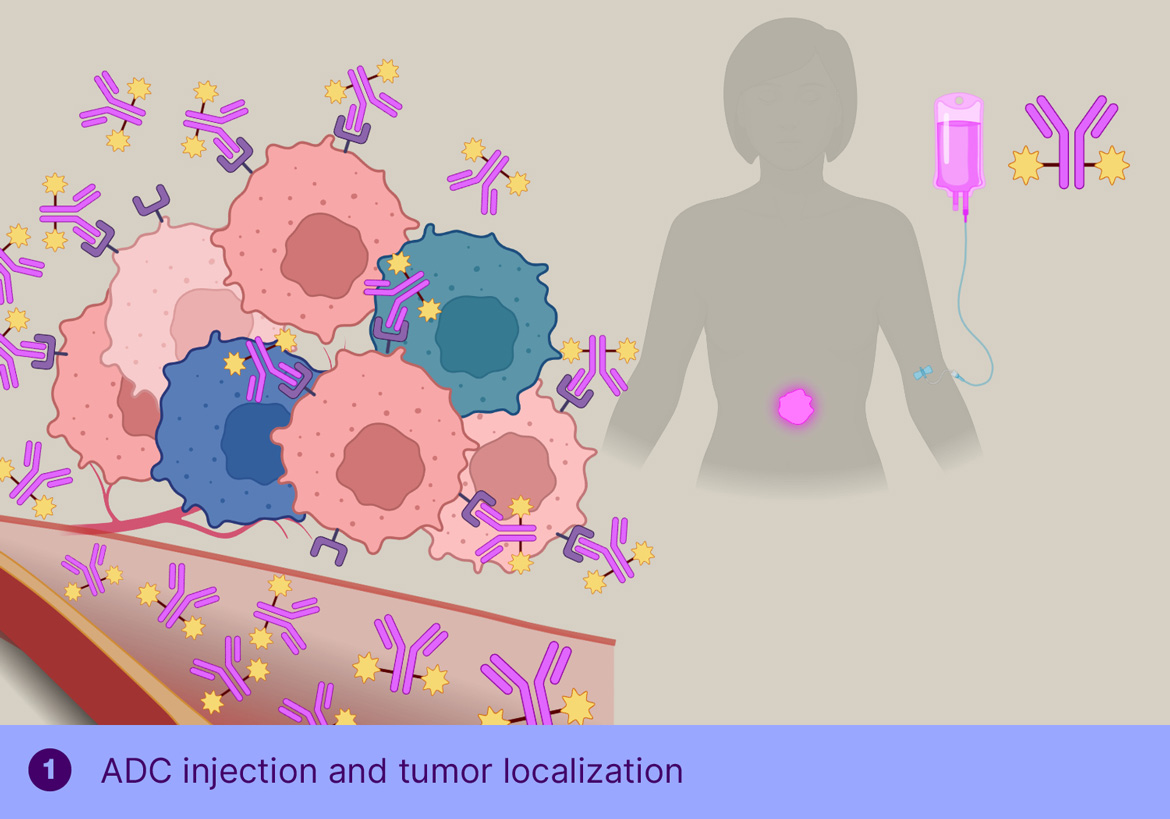
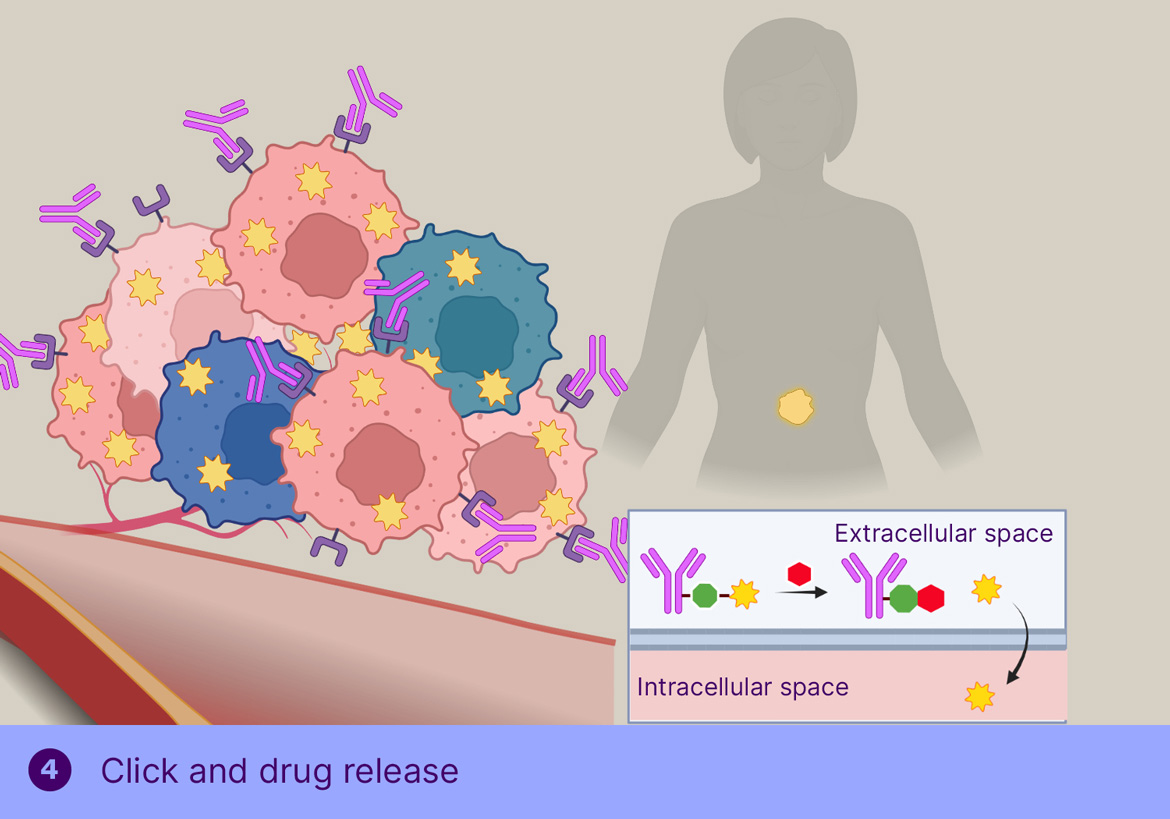
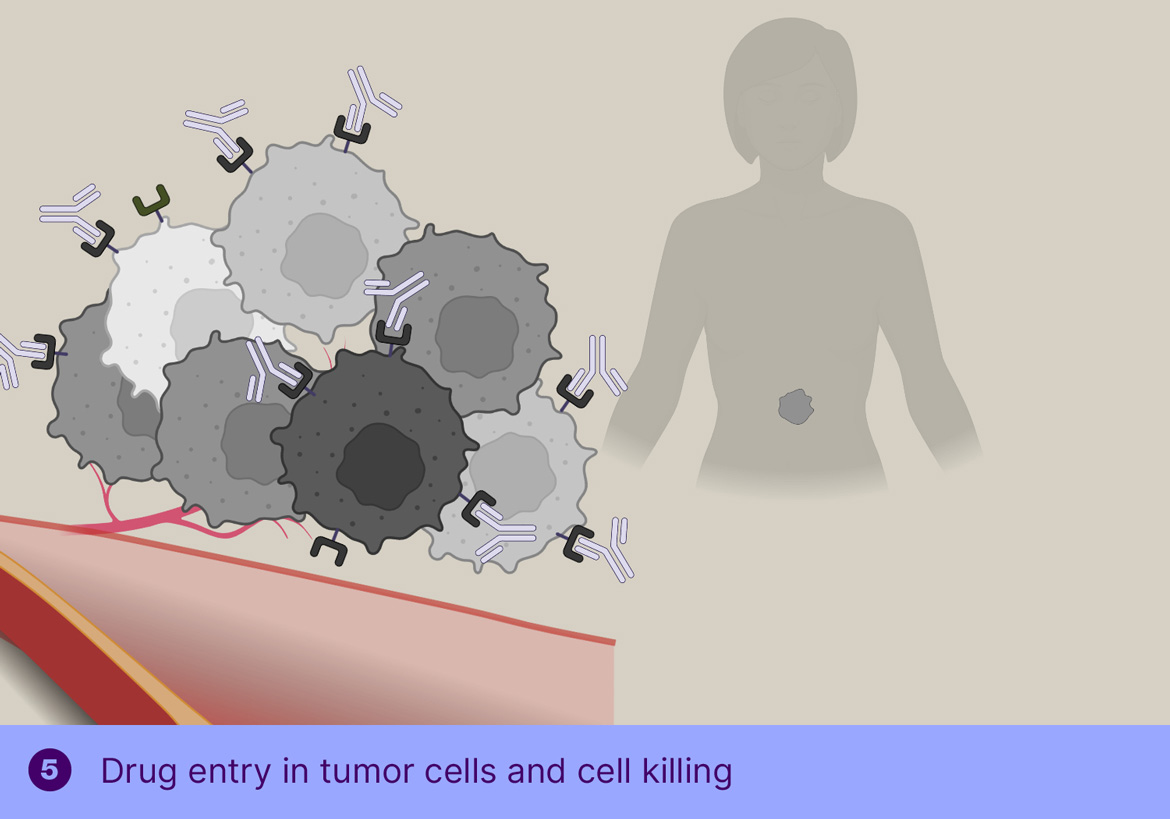
ADCs have long held great promise as powerful, highly targeted cancer therapies. The limitation of today’s conventional ADCs is that they rely on suitable internalizing targets, which are not necessarily expressed by every cancer type and cancer cell. Our ADC therapy, however, does not depend on internalizing targets as it is based on chemically controlled release of the payload in the tumor micro-environment, thereby killing heterogenous tumors where not every cell expresses the target. In addition, the stability of the linker in the absence of the trigger has the potential to allow a further improved therapeutic index. Importantly, our unique approach to targeting non-internalizing receptors also offers the opportunity to address a whole new cancer target landscape, especially in solid tumors.
Tagworks’ platform represents an opportunity to expand both the therapeutic index and reach of these therapies to additional tumor types, and to make a truly transformative impact on patients not served by existing therapies.
Click-Cleavable ADCs
Lead ADC Program: TGW101
Our lead program, TGW101, targets TAG72, a clinically validated, non-internalizing, pan-carcinoma target that is widely expressed in a range of epithelial-derived human cancers such as breast, colorectal, stomach, lung, pancreatic, prostate, and ovarian cancers. As it is relatively stable on the extracellular cell membrane, it has so far not been effectively addressed with current ADC technologies.
TGW101-ADC consists of a TAG72-binding diabody conjugated with TCO-linked monomethyl auristatin E (MMAE) toxin. MMAE has a well understood and manageable safety profile, and a well-documented bystander effect.
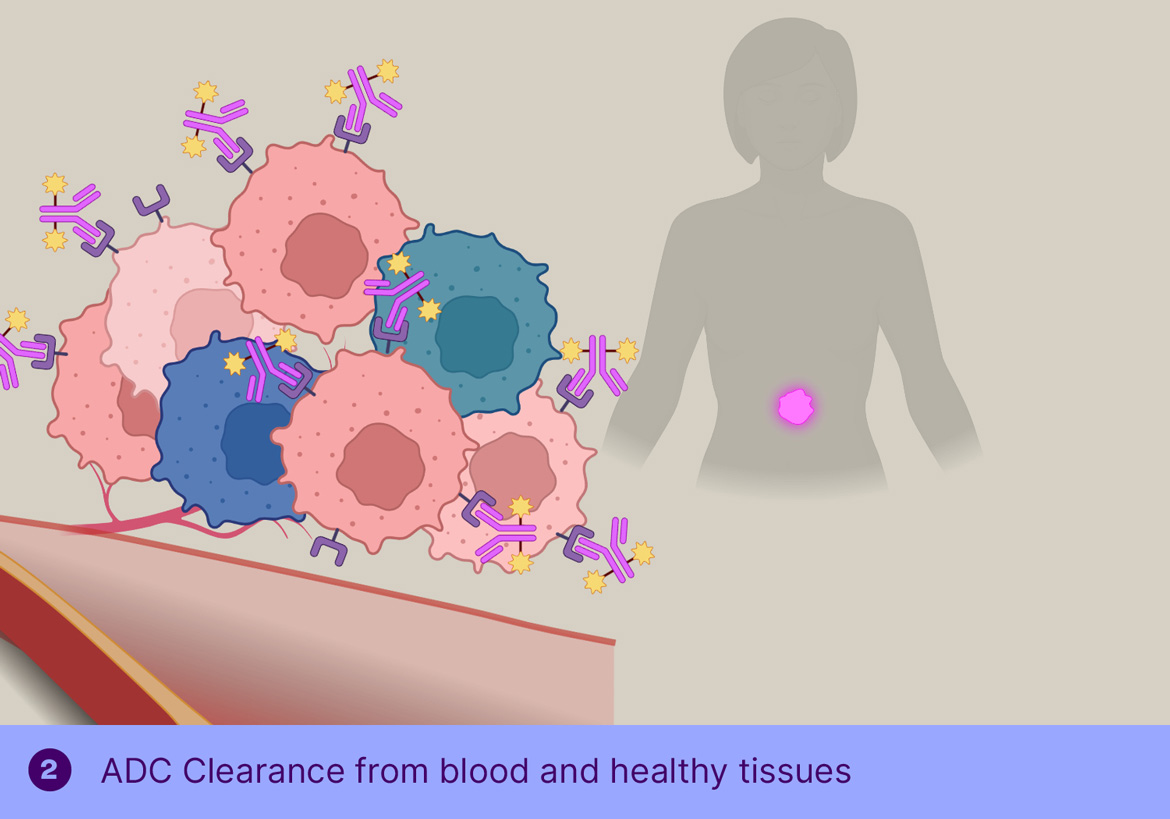
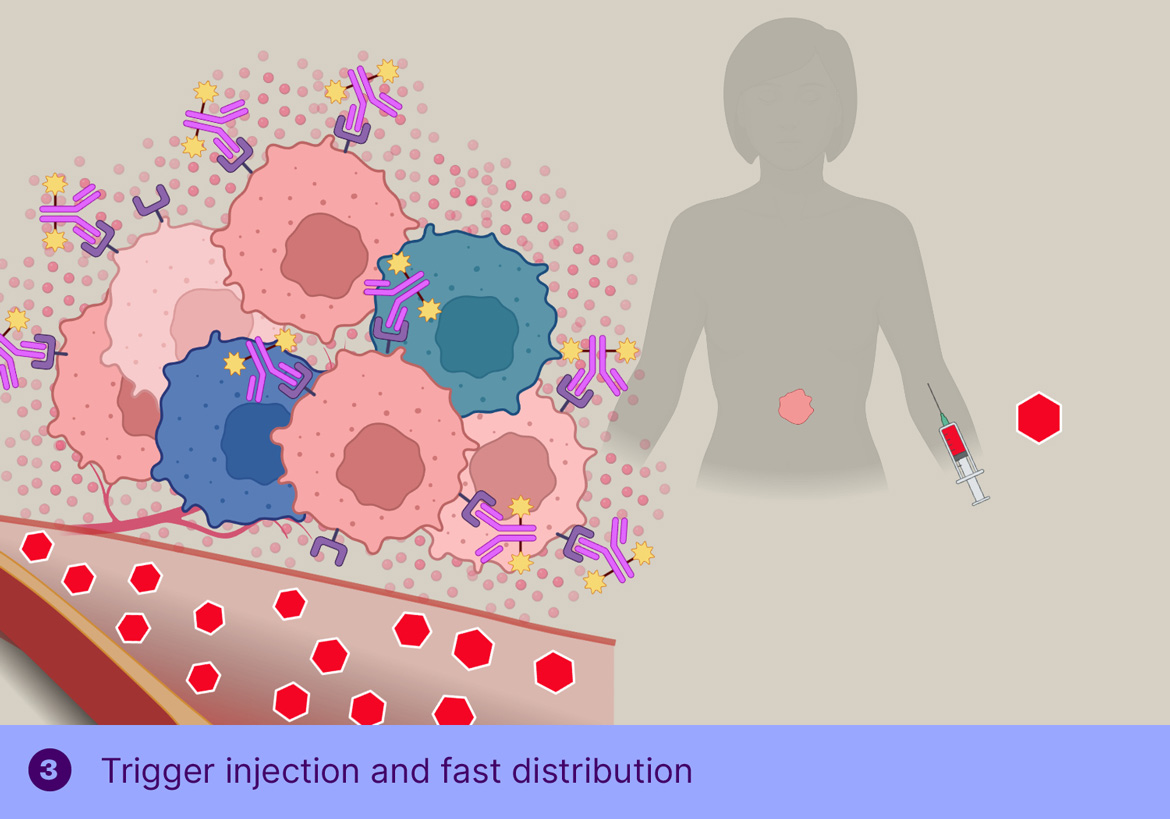
In TGW101 therapy, the ADCis administered first and allowed to bind the tumor and clear from circulation, after which the trigger molecule is injected systemically, resulting in cleavage of the tumor-bound ADC and passive distribution of MMAE into the surrounding tumor cells.
Our preclinical research indicates that on-tumor activation of TGW101-ADC leads to high and sustained concentrations of auristatin within the tumor microenvironment, affording a strong therapeutic effect, while mice that received an analogous protease-cleavable ADC progressed nearly as fast as the control group.
We are currently evaluating this program in IND-enabling studies.
Click-Cleavable Radiotherapeutics
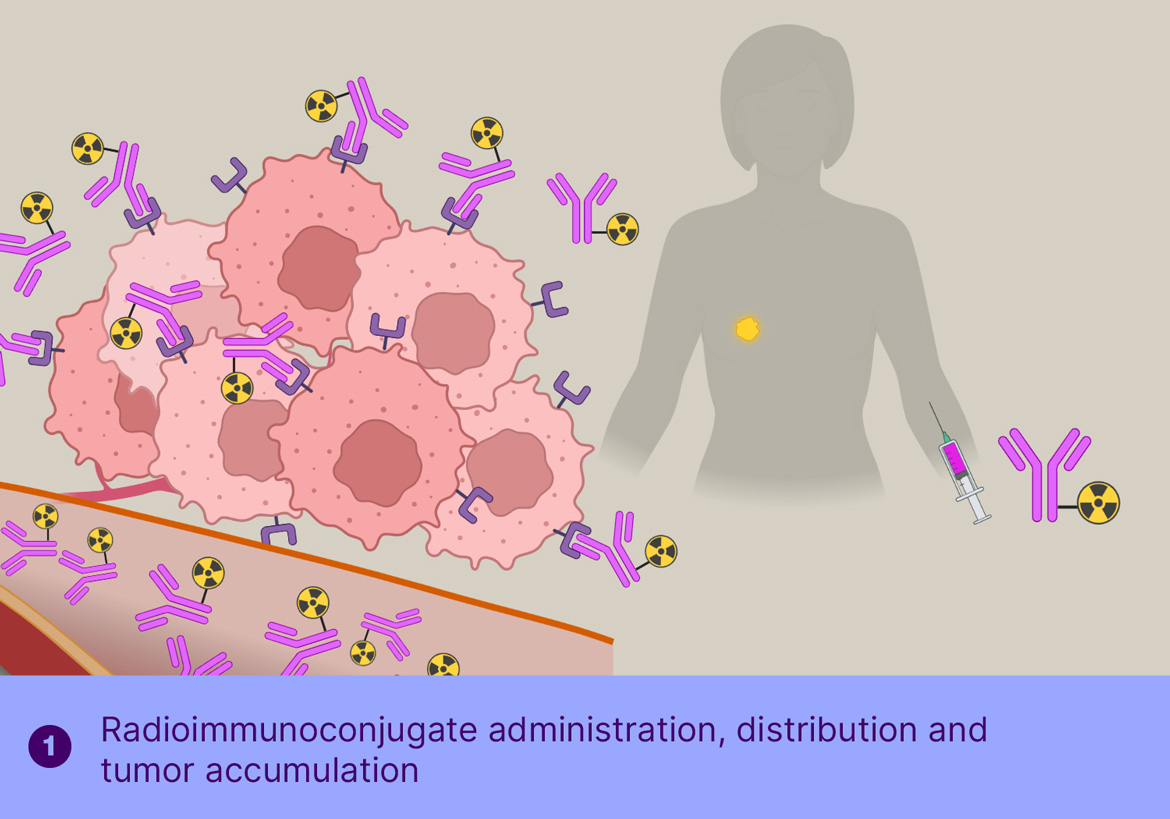
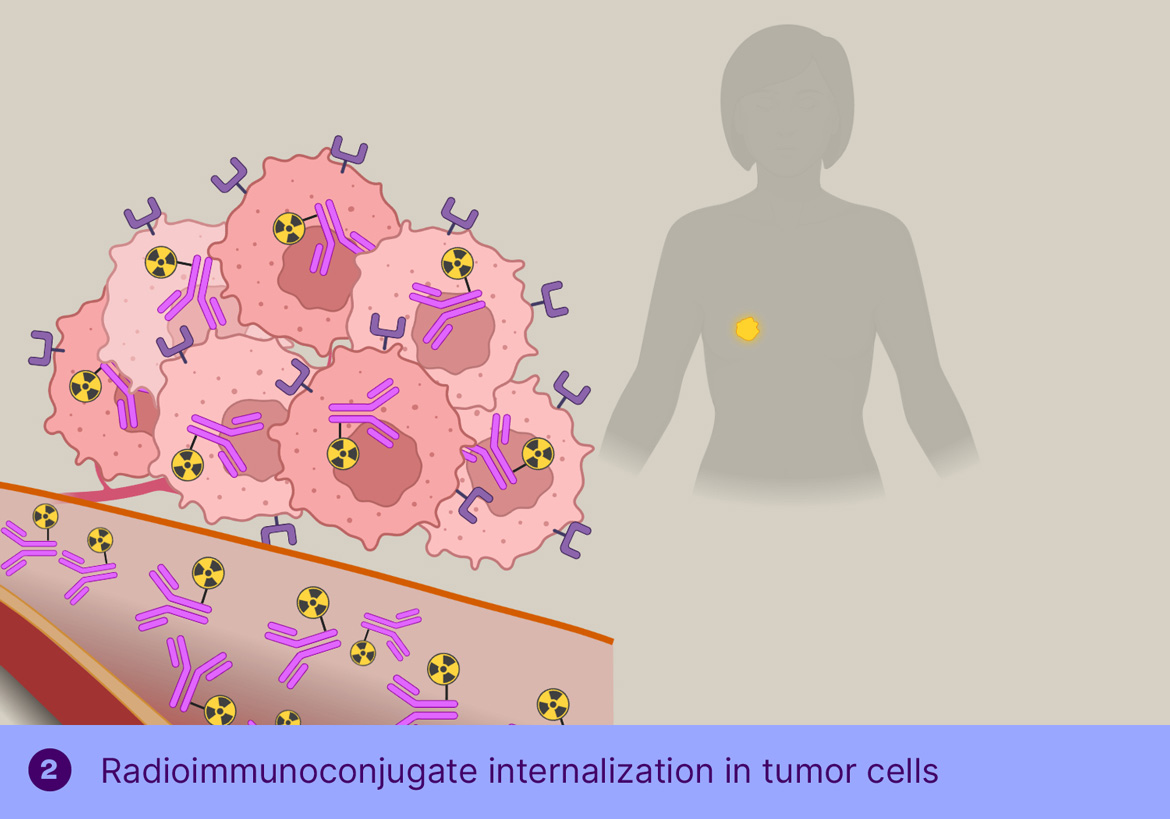
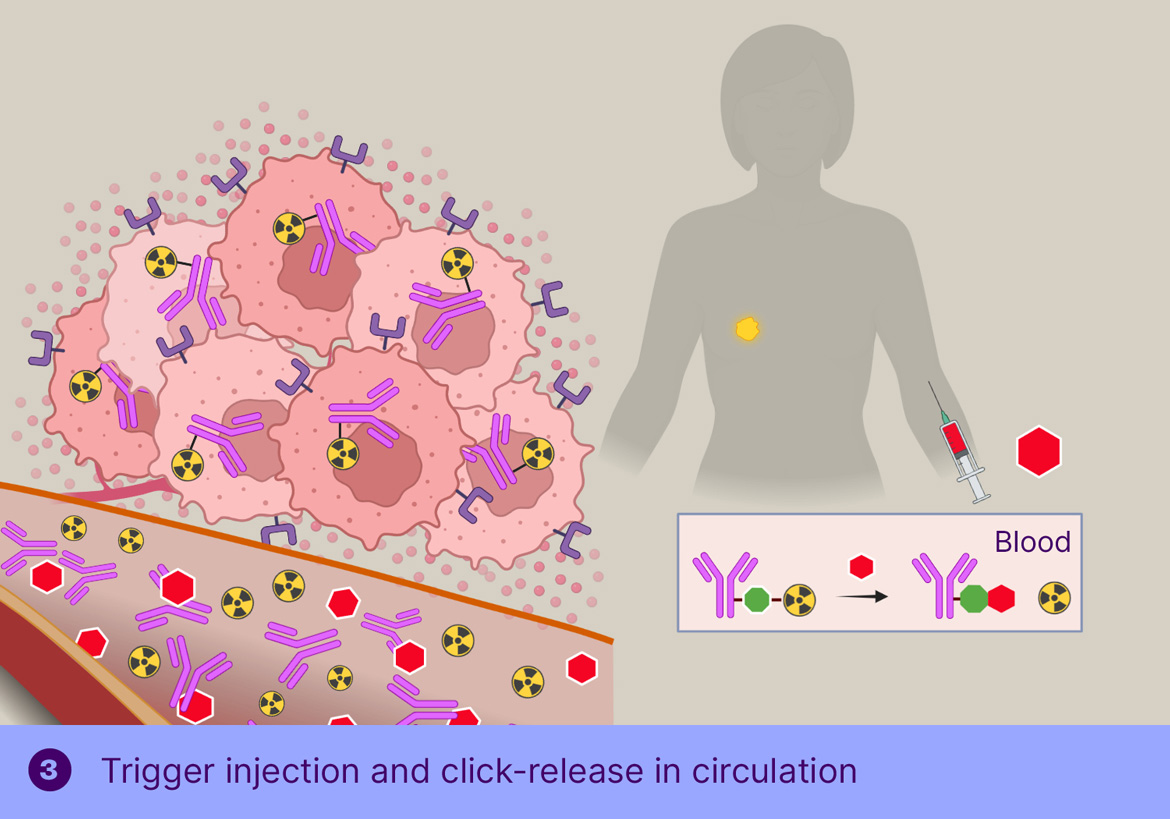
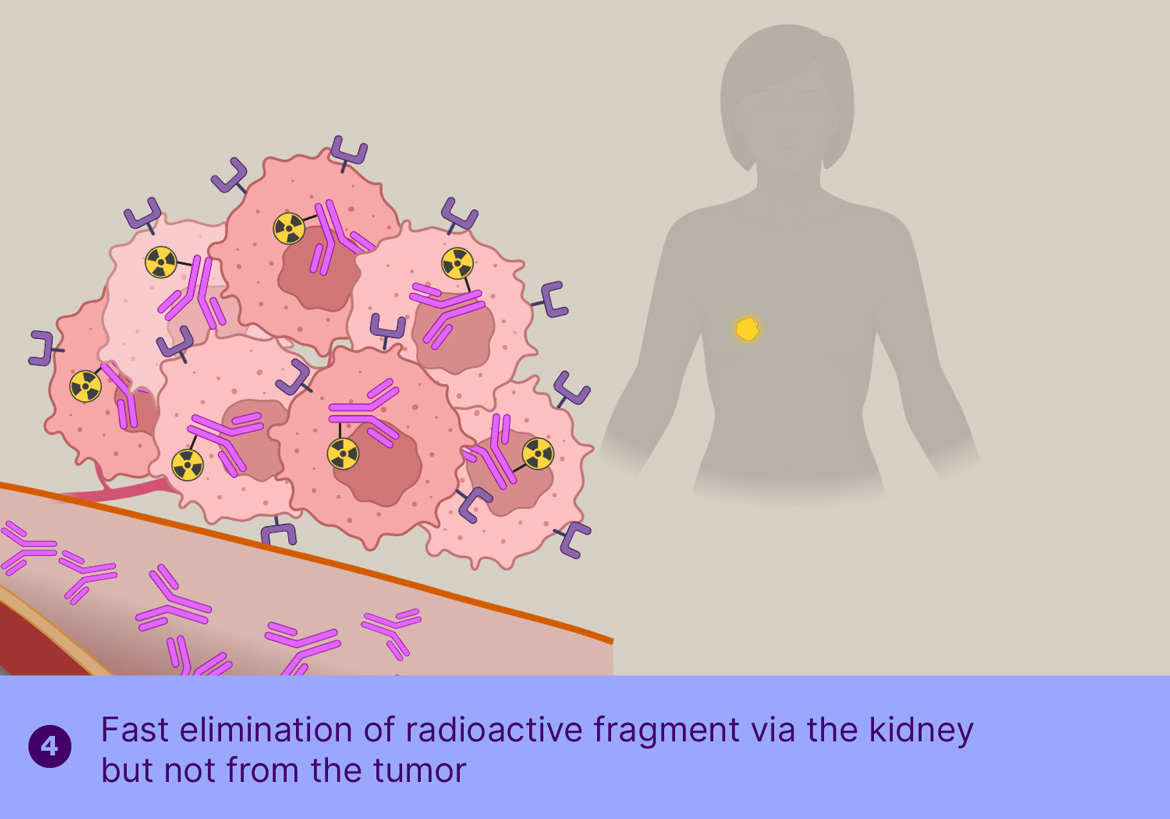
The main challenge for radioimmunotherapy is the long blood circulation of the radiolabeled antibodies, leading to high radiation doses to the bone marrow, limiting the dose that can be safely administered, thereby limiting the therapeutic effect. To overcome this, Tagworks is employing the Click-to-Release platform for the selective liberation and renal clearance of the radiolabel from freely circulating antibody once sufficient radiolabeled antibody has localized in the tumor cells.
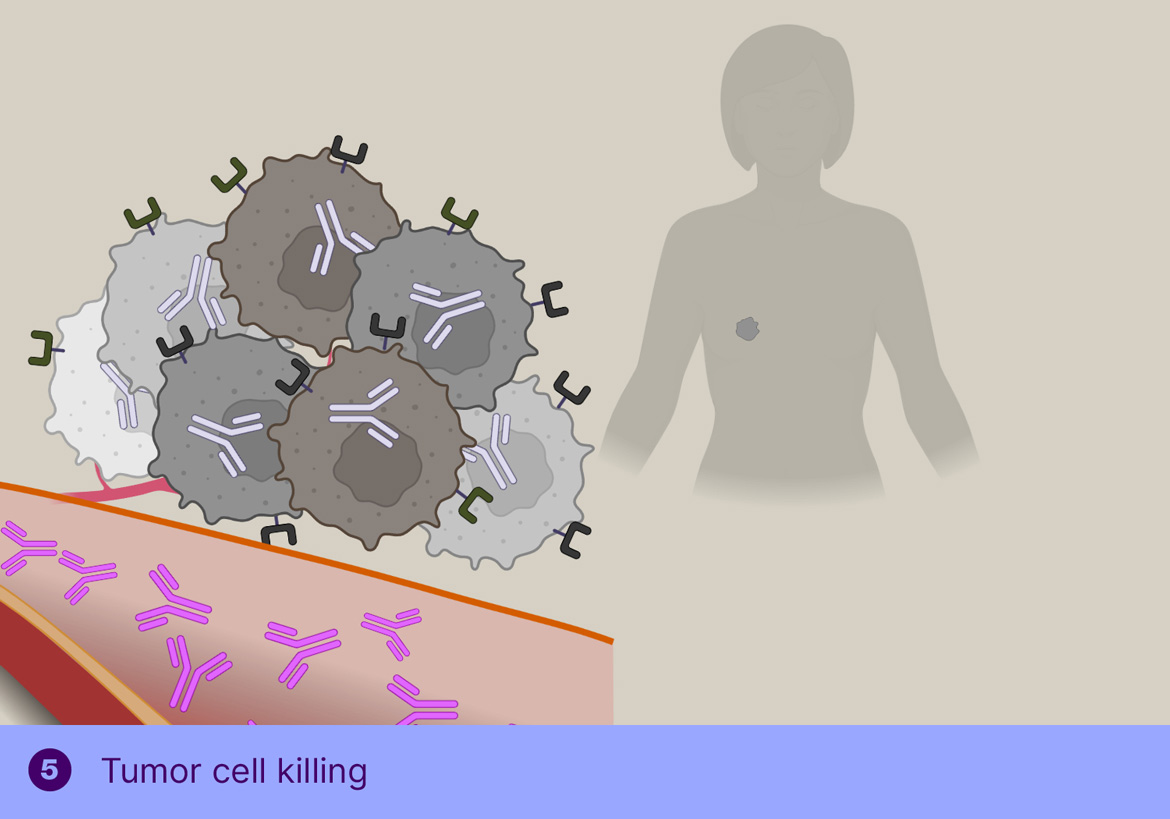
This off-target deactivation approach is designed to improve target-blood ratios of radiolabeled antibodies targeted to efficiently internalizing receptors. After sufficient tumor binding and internalization has occurred, the trigger is injected, which is not cell-permeable and will therefore only cleave the linker of the freely circulating antibody, resulting in rapid clearance of the cleaved radioactive fragment through the kidneys and a boost of the tumor-blood ratio. Tagworks discovery program includes a click-cleavable radioimmunotherapeutic for HER2-positive breast cancer.
Click-Cleavable Radiotherapeutics
Curious about what we do? Get in touch!
Contact
T +31 85 800 8550
info@tagworkspharma.com
Address
Tagworks Pharmaceuticals BV
Toernooiveld 1
6525 ED Nijmegen
The Netherlands
Stay updated
© Tagworks Pharmaceuticals, 2025 | Terms of use | Design by Ape to Zebra